First Steps
Feeling overwhelmed by all the information and preparation that goes into pursuing parenthood through sperm donation? We’re here with answers to your questions, helpful resources and any additional support you may need as you take your first steps on this amazing journey.
Family Resources

Frequently Asked Questions
Qualifications
Since 1975, we have provided guidance and expertise for those ready to fulfill their dreams of starting or growing a family. We let you choose from the industry’s most selective, most tested, and most successful donors.
Selective
Our donors are chosen with you and your future family in mind. All donors entering the program after 2017 must pass a rigorous screening process, which includes personality and behavioral evaluations.
Tested
We evaluate all donors using comprehensive carrier testing covering hundreds of genetic conditions. Testing panels are regularly updated to reflect the latest advancements in genetics.
Successful
Xytex prepares all vials with 0.5 milliliters of specimen, prepared to contain a specific number of motile sperm cells. MOT refers to the total number of motile sperm cells in 1 milliliter of sample. So the minimum number of motile sperm in each vial is one half of the MOT number.
Here are two examples:
An unwashed vial (25MOT) that is loaded with 0.5 milliliters of sample will carry a minimum of 12.5 million motile sperm.
A washed vial (20MOT) that is loaded with 0.5 milliliters of sample will carry a minimum of 10.0 million motile sperm.
To view a current list of licenses, registrations and certifications, please visit our Licenses page.
We also adhere to the regulations regarding importation of products by the governments of Switzerland, Australia, and the United Kingdom (HFEA), as well as Health Canada, a federal governing board of Canada.
Your fertility journey is a very personal matter, and we respect your privacy. The information you share with us is held in strictest confidence, unavailable to anyone, other than your health care provider, without your prior permission. Your information will not be given or sold to any other company or institution. Likewise, we respect donor privacy and only release information about our donors with the donor's prior written consent. Encryption of a client's financial information is provided through Paya and Merchant Lynx.
Donors
At Xytex, we recognize how important it is for families to connect - and we’re always looking for ways to make those connections stronger. Your feedback on Famlink showed us new opportunities to better serve you, and we appreciate your support throughout this process.
We’re excited to share that we’re developing a new system: a secure database that puts you in control of how you connect with others. By allowing you to share your own contact information and choose the platforms you prefer, we’re taking moderation out of the equation - so your connections are more private and more organic.
Our goal is to have this resource ready by Summer 2025. As an employee-owned company, we believe in offering personalized experiences and genuine support to every individual on a family-building journey. Because of that, this initiative is a top priority, and we’re dedicating the time and care necessary to ensure it’s both secure and user-friendly.
Thank you for being part of the Xytex family. We look forward to providing this new platform that helps you stay connected in the ways that matter most.
A retired donor is no longer actively donating. This category of donors may or may not have remaining inventory available for purchase.
Selecting a sperm donor is a personal and sometimes complex decision, and we're here to support you throughout the process. Xytex strongly recommends consulting with your healthcare provider and a genetic counselor for individualized medical guidance as you make your selection.
To begin, create an account to access detailed donor profiles and additional selection tools. We suggest starting by identifying your key priorities—such as genetic screening, physical traits, education, or medical history—and using our search filters to help narrow your options.
If you have questions or would like more personalized support, we offer complimentary Zoom consultations with a member of our team.
Once you have selected your donor, you can place an order online or call us at 706-733-0130. Our team will be happy to guide you through the ordering process.
As of January 2019, Xytex no longer accepts Anonymous Donors into the donor program.
Clients who have reported births from an anonymous donor may still purchase vials from the remaining inventory, subject to availability.We understand the importance of a child having the opportunity to learn more about their sperm donor. That’s why Xytex offers Identity Disclosure Donors—donors who have agreed to allow their identifying information (such as full name and last known contact details) to be shared with the donor-conceived individual once they turn 18.
To be eligible to receive this information, the child’s mother—the recipient of the donor sperm—must sign the Identity Disclosure Agreement and report the child’s birth to Xytex. These steps allow us to verify the child's eligibility and ensure accurate records are maintained for future disclosure.
While a donor’s participation in the Identity Disclosure Program reflects a willingness for transparency, it does not guarantee any form of contact or relationship. It simply permits the release of identifying information when the child becomes of age and requests it.
Our online donor database is regularly updated to reflect changes in donor status and inventory. While we aim to show donors with current availability, inventory levels can fluctuate due to ongoing demand.
If a donor you are interested in is currently unavailable, we encourage you to check back regularly, as inventory may be replenished. New donors are added frequently, providing a wide range of options to support your family-building journey.
In some cases, retired donors may still have limited inventory available. If so, this will be reflected on their profile.
Please note that Sibling-Only Donors—those reserved for families who have previously reported a birth—require a documented birth on file before additional vials can be purchased.
We require both child and adult photos for our donors. However, you may come across a few donors who joined the program before this requirement was implemented, thus only childhood photos may be available.
Genetic factors can be an important part of the donor selection process. For more information about genetics and genetic testing, please view our Genetics FAQ.
At Xytex, we take donor usage limits seriously to ensure ethical distribution and safeguard the well-being of donor-conceived individuals. Each donor's availability is limited based on both the length of time he remains in the program and the number and location of reported births.
We follow the recommendations of the American Society for Reproductive Medicine (ASRM). This guideline reflects Xytex’s commitment to supporting the long-term interests of donor-conceived people by helping to limit the number of families per donor. It aligns with industry standards and is part of our effort to promote responsible donor use across regions.
To comply with these standards, we:
monitor the geographic distribution of donor vials.
ask clients to report births so we can keep accurate records. Based on the factors listed above, access to a donor may be restricted for creating additional siblings.
Building a family looks different for everyone. Our donor options are designed to reflect your unique path and support the choices that matter most to you.
Exclusive Donors
These donors are available for purchase by a single Xytex family.XYlimited Donors
With limited inventory and a set purchase requirement, these donors are available to fewer families.
Whether you're exploring donor options, planning for future siblings, or navigating next steps—our team is here to offer guidance, clarity, and support tailored to your family-building goals.
Yes, reported pregnancies are listed on each donor’s profile. However, Xytex does not provide pregnancy success rates, as many factors—such as insemination timing, method, thawing process, and recipient fertility—can affect outcomes and are beyond our control.
A donor without reported pregnancies is not necessarily infertile. This may simply indicate they are new to the program. All Xytex donors undergo rigorous screening, including laboratory testing and physical exams, and are regularly monitored to ensure the highest quality standards throughout their participation.
Quality Standards
The health and medical history of our donors are extremely important. While each donor is different, they all go through the same thorough screening process.
- Each candidate completes a medical history questionnaire (MHQ).
He will answer detailed questions about his health status and provide family medical history. - Each candidate provides a semen sample for analysis.
We assess sperm count, volume, motility and additional factors to determine quality of the donation. - Each candidate is appropriately vetted.
A Donor Coordinator interviews the candidate about his personal and family medical history. During this step, the donor also undergoes a background check and education verification process.* - Each candidate undergoes psychological assessment.
This process includes a complete behavioral analysis, psychosocial, and risk assessment completed by a clinical social worker and a licensed clinical psychologist.* - Each candidate undergoes laboratory testing and a physical exam.
Following the semen sample evaluation, blood and urine samples are taken during pre-screening and are periodically screened throughout continued sperm donation.
With active participation in the program, donors undergo a physical exam and blood draw every six months. Contingent on inventory demand, some donors may be screened more frequently. A donor who remains in good health can continue to participate in our program until he reaches his 41st birthday, or until he reaches our limit of reported family units.
*Donors entering the program in 2017 or later have undergone background checks and psych-social evaluations.
- Each candidate completes a medical history questionnaire (MHQ).
While Xytex may, from time to time, get and share updated clinically significant medical and/or genetic information with Client and Client's Healthcare Provider, it is up to Client, before using any Specimens obtained from Xytex, to contact: (I) Client's Healthcare Provider; and (II) Xytex, for any such updated information of which Xytex may have become aware. Client acknowledges and agrees that under some circumstances, Xytex may have shared such updated information only with Client's Healthcare Provider, in which event, whether or not Client would be informed of such updated information would be entirely dependent upon Client's contacting Client's Healthcare Provider and receiving such updated information from Client's Healthcare Provider.
Although Xytex is not obligated hereunder (or otherwise) to disclose or share with Client or Client's Healthcare Provider any updated clinically significant medical and/or genetic information, in the event Xytex does share any such updated information, an experienced genetics counselor should be consulted to advise Client as to its potential significance. Xytex is not a medical provider and cannot provide medical advice.
We conduct laboratory testing and physical exams on all donors to assess their overall health. Additionally, we conduct genetic testing on all donors to help you confidently choose your family.
Screening looks for evidence of, the most common symptoms of a particular infection. Testing involves applying laboratory methods to biological specimens, such as blood, urine, or semen, to determine the presence of a specific infectious agent, such as a bacteria or a virus.
Fresh semen is unsuitable for sperm banking since it begins to lose fertility within an hour or two of ejaculation. Because a sperm bank is expected to have semen available from a diverse set of donors with different physical characteristics and different talents, it is necessary to store sperm from the time of collection until it is needed. We accomplish this through a unique freezing process called cryopreservation, which gives semen an extended survival period. It is the only way sperm can be shipped from our bank to a client, no matter where they live. Once thawed, cryopreserved sperm can be as fertile as fresh sperm.
Sperm is also cryopreserved to allow a quarantine period and retesting of various diseases, such as HIV which causes the potentially-lethal AIDS infection. Cryopreserved sperm must be stored for a minimum of six (6) months before repeat testing of the donor. Repeat testing includes HIV 1/2 + O antibodies, HTLV I & II antibodies, hepatitis B surface antigen, hepatitis B core antibody, hepatitis C, HIV/HCV/HBV NAT, TP-PA, and cytomegalovirus (CMV) antibodies. Donors are also tested for chlamydia and gonorrhea.
Scientists believe the potential for long-term storage is indefinite, assuming adequate maintenance of low temperatures.
Before 10/01/2017, Xytex semen was cryopreserved in sterile-filtered Test Yolk Buffer (Irvine Scientific, Santa Ana, CA), composed of buffers (TES and Tris), sodium citrate, fructose, gentamicin sulfate, glycerol, and heat-activated egg yolk from specific pathogen-free laying flocks. Detailed product information can be found at https://www.irvinesci.com/assisted-reproductive-technology/sperm-preparation-media/freezing-medium-tyb-with-glycerol-gentamicin.html
Between 10/01/2017 and 05/01/2018; Units were cryopreserved with Sperm Maintenance Medium (Irvine Scientific, Santa Ana, CA). The medium does NOT contain egg yolk or antibiotics. It does contain HEPES and human serum albumin.
As of 05/01/2018, donor sperm is cryopreserved in ArcticTM Sperm Cryopreservation Medium (Irvine Scientific, Santa Ana, CA). The medium does NOT contain egg yolk or antibiotics. It does contain HEPES, MOPS, and human serum albumin. Detailed product information can be found at http://www.irvinesci.com/products/90170-arctic-sperm-cryopreservation-medium.
Comparing human births resulting from thawed semen to non-assisted pregnancies shows no difference in the rate of birth defects or miscarriage.
Cytomegalovirus (CMV) is a common virus that infects approximately 70% of persons in the United States by age 45 and more than 90% by age 80.? In most cases, initial infection results in no symptoms, though some people experience a mild illness, similar to the flu or mononucleosis.? After the initial infection, the virus remains dormant (latent) in the body.? Reactivation of infection typically occurs in persons with compromised immune systems.
If a woman who has never been exposed to CMV becomes infected with the virus during pregnancy, there is a 40% risk of fetal infection.? In contrast, the risk of fetal infection is only 1% in women with previous CMV exposure.? Fetal infection with CMV can result in miscarriage and serious developmental defects in approximately 10% of cases where primary infection occurs during the first 14 weeks of pregnancy.
Xytex tests all donors for CMV and includes each donor’s status in his profile. CMV status is based on testing blood for antibodies (IgG and IgM) to the virus. CMV-negative donors have no IgG or IgM antibodies. CMV-positive donors have IgG antibodies that persist for life, but the pattern of antibodies determines whether infection is latent (inactive) or active. Xytex does not accept sperm from donors whose test results suggest active or recent CMV infection (IgM-positive). Sperm from donors with latent infection (IgG-positive, IgM-negative) is considered to be safe.
Knowing your CMV status can help navigate your fertility journey. While it might seem reasonable to accept only CMV-negative donors, such a restriction would reduce the donor pool by more than half, leaving women with fewer choices. Xytex encourages clients to discuss CMV with their healthcare providers, who may recommend CMV testing. Providers often recommend that CMV-negative patients use sperm from CMV-negative donors.
Process
Xytex relies on clients to report all births resulting from donor sperm use. Births should be submitted through your Xytex website account portal.
Timely birth reporting is essential to:
Maintain accurate tracking of family units per donor
Ensure continued access to the donor for clients building genetically related families, pending vial availability
Allow ongoing access to the donor’s profile and pertinent donor updates, such as medical or genetic information
Uphold the integrity, safety, and compliance of our donor program
Your cooperation helps protect donor-conceived children, supports responsible family planning, and strengthens the transparency and long-term success of the donor community.
ART vials are often used in IVF (in vitro fertilization) – sperm and egg are combined in a petri dish for fertilization.
An unwashed vial is frozen in its most natural state, allowing sperm to remain in its natural seminal fluid. Unwashed vials are used for ICI (intracervical insemination) – sperm enters through the cervix, much like in sexual intercourse.
A washed vial separates the sperm from its natural seminal fluid and is frozen in preserving fluid. Washed vials are used for IUI (intrauterine insemination). While we offer washed vials prepared for IUI treatment, we also offer unwashed vials, which are suitable for IUI, but require washing by the inseminating clinic.
*Please consult your Clinic for the appropriate vial type for your procedure.
Please also see this helpful video!
First, create an account and browse through our catalog of sperm donors. With our interactive features, you can view child and adult photos and gain insight from donors’ personal essays. When narrowing your search, we urge you to choose several of your top picks and rank them in order of preference.
When you’re ready, give us a call, and we will check availability of your top picks, making every effort to secure your first-choice. However, due to normal fluctuations in our inventory, this is not always possible. If you'd like to use the same donor for future children, we recommend buying additional vials during your initial purchase.
We also recommend consulting your healthcare provider when choosing a donor. They will be able to help you navigate things like expected day of ovulation and the number and type of vials needed for your procedure. We strongly recommend planning to have your vials delivered a day or two prior to ovulation.
Xytex is unable to facilitate the transfer of ownership of your vials to a third party. Xytex has a strict policy prohibiting this type of transfer, as Xytex cannot guarantee the quality or integrity of the vials once shipped from our facility or another designated storage facility. In furtherance of this policy, clients complete a service agreement that states they are purchasing the vials for their own use or use by a partner and are not permitted to transfer them to any other entities. By following these policies, Xytex can maintain vial safety, compliance, international family limits and properly observe applicable laws.
An unwashed vial is frozen in its most natural state, allowing sperm to remain in its natural seminal fluid. A washed vial separates the sperm from its natural seminal fluid and is frozen in a preserving fluid. Removing seminal fluid helps minimize cramping that can result from prostaglandins, the hormones that cause the uterus to contract.
Washed vials should be used in IUI procedures, as unwashed vials injected into the uterus can cause cramping. In addition to being extremely painful, it will most likely result in the loss of injected sperm. Both washed and unwashed samples can be used for ICI or intracervical inseminations. ART samples are processed in exactly the same way, with the only difference being the minimum number of motile cells.
We accept wire transfers and all major credit cards.
If your vials are being stored at Xytex or CANAM:
With a purchase of 6 or more vials, the 1st swap is complimentary. Each additional swap is $895.
With a purchase of 5 vials or less, there is a first swap fee of $150. Each additional swap is $895.
Once vials have left Xytex or an approved storage facility, they are ineligible for returns or exchanges.
The easiest way to ensure access to the same donor for future inseminations is to buy additional vials during your initial purchase. You can request to store them at our facility until needed for treatment.
Sign in to “My Account” and visit the Birth Report tab.
Since 1975, we have provided the expertise your patients need to fulfill their dreams of starting or growing a family. We let your patients choose from the industry’s most selective, most tested, and most successful donors.
Selective
Our donors are chosen with your patient and her future family in mind. Since January 2017, all donors have endured a rigorous screening process, which includes personality and behavioral evaluations.
Tested
We evaluate all donors for hereditary conditions using an extensive medical history questionnaire and carrier testing for genetic conditions.
Successful
Our donors have a minimum 12.5 million motile sperm per 0.5 mL in unwashed vials and 10.0 mL motile sperm per 0.5 mL in washed vials.
To view a current list of licenses, registrations and certifications, please visit our Licenses page.
We also adhere to the regulations regarding importation of products by the governments of Switzerland, Australia, and the United Kingdom (HFEA), as well as Health Canada, a federal governing board of Canada.
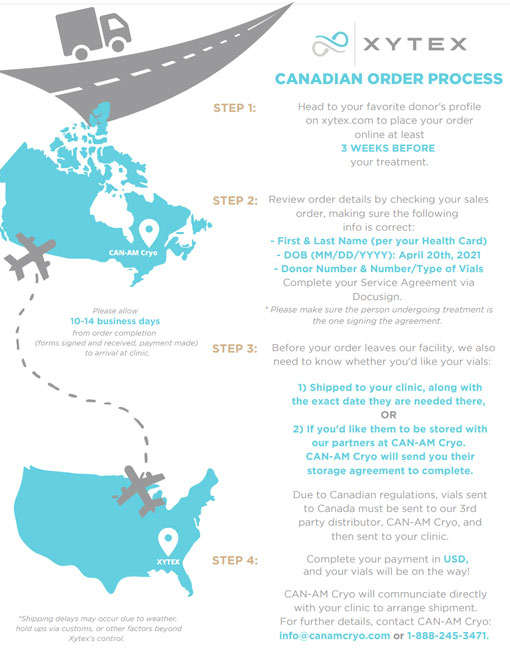
Xytex has partnered with CAN-AM Cryo www.canamcryo.com to import, store and distribute Xytex donor sperm. CAN-AM works closely with the Client Relations team at Xytex and will arrange for your Xytex donor vials to safely arrive at your clinic in Canada or store them until you need them for your procedure.
*Please note that as per Health Canada Regulations, Can-Am Cryoservices Corporation has notified Health Canada as required under s.18 of the Safety of Sperm and Ova Regulations for the activities of importation and distribution of semen which has been processed by Xytex Corporation
Shipments to Canada typically take 10-14 business days. If you need expedited shipping, you can request it, though there is a $95 fee charged by CanAm, our Canadian importing partner. Please note that expedited shipping is not guaranteed. For any additional questions or inquiries regarding your shipment, you can contact CanAm directly at 888-245-3471.
At Xytex, we are committed to providing a personalized client experience every step of the way. Our services include:
Access to our experienced Client Relations Team for individualized guidance and support.
A complimentary 30-minute Zoom consultation to answer your questions, walk you through our process, and help you feel confident in your next steps.
Secure 30-day access to explore donor profiles at no cost, so you can begin your search comfortably and at your own pace.
Client portal access to manage storage, place orders, and send secure messages directly to our team.
Flexible shipping options, including delivery to clinics or your home (with certain state restrictions), for your convenience and privacy. (options limited to US clients)
Education on donor selection, vial types, and storage, tailored to your needs and circumstances.
If you have questions or need support at any time, our dedicated team is here to help.
Canadian Compliant means a donor’s units are compliant with the requirements of Health Canada, including relevant sections of the Assisted Human Reproduction (AHR) Act and the Safety of Sperm and Ova Regulations. The Safety Regulations are intended to prevent the spread of infection to recipients of donor sperm by screening and testing donors for relevant infectious diseases.
As a registered processor and distributor of donor sperm, Xytex ensures all donor sperm made available to Canadian clients is Canadian Compliant. Inventory changes quickly and often, so please call or email the Client Relations team for availability and to obtain updates on possible future releases of additional units for a particular donor.
Our website is designed so you may perform donor selection searches independently and at your own pace.
The complete listings for donors that we currently have in our inventory may be found using the Search Donors page and are updated to reflect current available inventory.
Since November 15, 2021, Quebec’s Régie de l’assurance maladie du Québec (RAMQ) Medically Assisted Reproduction (MAR) Program has provided financial support to eligible residents undergoing fertility treatments. As part of this initiative, the program covers a portion of the cost of donor sperm vials and shipping, helping to make assisted reproduction more accessible and affordable.
This coverage is available to Quebec residents who meet the province’s eligibility requirements, including having a valid RAMQ health insurance card and receiving treatment through an approved fertility clinic. The goal of the program is to reduce out-of-pocket expenses for individuals and couples pursuing family-building through donor sperm, particularly in medically supported settings such as IUI or IVF.
Patients approved for RAMQ MAR benefits must still work with a participating clinic to process orders, submit claims, and coordinate eligible services. Xytex is proud to support clients using RAMQ by working closely with approved clinics to ensure smooth ordering and delivery.
If you believe you may qualify for RAMQ coverage, we encourage you to speak with your fertility clinic for guidance on eligibility, application steps, and coordination with Xytex.
Eligibility for the RAMQ MAR program is determined by the Government of Quebec. For full program details and to verify your eligibility, please visit the official RAMQ website.
For details on the program and to check eligibility, visit this page.
Can-Am is a registered distributor participating in Quebec’s RAMQ Medically Assisted Reproduction (MAR) Program, allowing eligible patients to pay only the uncovered portion of their donor sperm order upfront. The remaining approved costs are billed directly to RAMQ, streamlining the process for patients receiving treatment in Quebec.
Xytex has a select group of donors available for direct purchase on the Can-Am website, making them immediately eligible for RAMQ coverage. This option offers a simplified path to reimbursement for those working within the program.
Patients may also choose a donor from the full Xytex donor database; however, these orders are considered self-pay and are not processed directly through RAMQ. In these cases, it is the responsibility of the patient to determine eligibility and submit reimbursement requests through the appropriate RAMQ channels.
For questions about which option is right for you, we recommend consulting your fertility clinic or reaching out to Can-Am for assistance.
To place an order through RAMQ:
1. Select Your Donor: Select your donor in one of two options:
- On the Xytex website, you can filter your donor search by RAMQ donors. Choose your donor then click the “Order with RAMQ” button; this will navigate you to the Can-Am website.
- On the Can-Am website, add your chosen donor from Xytex to your cart.
2. Select “RAMQ” as Payment Method: Choose “RAMQ” at checkout. You will not be charged upfront; RAMQ will handle the eligible portion of the costs.
3. Important Coverage Details: Only one unit per cycle is covered, with a maximum of six cycles.
Quebec residents eligible for the RAMQ Medically Assisted Reproduction (MAR) Program may purchase donor sperm from a select list of RAMQ-covered donors through Can-Am. However, participants also have the flexibility to choose from Xytex’s full, expansive donor catalog at www.xytex.com if they wish to explore additional options.
If you select a donor directly from Xytex, please note the following:
Xytex is a U.S.-based company, and all orders are processed in U.S. dollars (USD).
You will be considered a self-pay client and are responsible for paying the full cost of your vials and shipping at the time of purchase.
Xytex will provide an official invoice as proof of purchase, which can be used to seek reimbursement.
Depending on your clinic’s policy, you may be reimbursed either through your fertility clinic or directly by the RAMQ.
It is the client’s responsibility to confirm eligibility for RAMQ reimbursement and to initiate the reimbursement process with their clinic or the government. Not all clinics participate in direct RAMQ coordination for third-party donor orders, so it is important to speak with your clinic prior to ordering.
Clinics currently known to support this process include: Montreal Fertility, Ovo Fertility, and McGill Fertility. Clinic participation may vary—please confirm with your provider for the most accurate and up-to-date information.
If you need help understanding the ordering process or navigating reimbursement requirements, our Client Relations team is happy to assist.
Can-Am provides all necessary invoices and receipts at the time of order placement. These documents can be used to support your claim and submitted if RAMQ requests additional verification. In some cases, your fertility clinic may also issue a certificate of eligibility, if required for your application.
For clients purchasing directly through Xytex, a proof of purchase will be provided. This documentation may be used to initiate a self-submitted reimbursement request with RAMQ if applicable.
Yes, the RAMQ Medically Assisted Reproduction (MAR) program includes specific limits on the number of donor sperm units covered per patient. These limits are designed to ensure equitable access to fertility treatments while managing healthcare resources responsibly.
The exact number of covered units may vary depending on individual circumstances, such as the type of treatment (e.g., intrauterine insemination or in vitro fertilization), patient eligibility, and clinical recommendations. It is important to consult your fertility specialist or clinic to understand the coverage details applicable to your treatment plan.
For the most accurate and personalized information, please refer to the RAMQ guidelines or speak directly with your healthcare provider.
For help with the RAMQ order process, please contact the Can-Am customer support team or your clinic’s billing office. Xytex’s Customer Relations Team is also available to answer questions.
Once you have selected RAMQ as your payment method and your order has been processed through the program, cancellations or modifications may be limited due to the administrative and billing procedures involved. Because orders are coordinated directly with your fertility clinic and the RAMQ system, changes could affect coverage eligibility and billing.
If you need to cancel or modify your order, please contact your fertility clinic as soon as possible. They will work with the distributor and RAMQ to determine if adjustments can be made.
It is recommended to carefully review your order details and confirm with your clinic before finalizing your purchase using RAMQ coverage.
Depuis le 15 novembre 2021, le programme de procréation médicalement assistée (PMA) de la RAMQ couvre une partie du coût des fioles de sperme de donneur et des frais d'expédition pour les patients admissibles. Le programme soutient les résidents du Québec qui suivent des traitements de fertilité en réduisant les frais qu'ils doivent débourser pour ces services.
Les critères d'admissibilité au programme RAMQ PMA sont définis par le gouvernement du Québec. Pour plus de détails sur le programme et pour vérifier l'éligibilité, visitez le siteweb.
Can-Am est un distributeur agréé participant au programme de procréation médicalement assistée (PMA) de la RAMQ au Québec, ce qui permet aux patients admissibles de ne payer d’avance que la portion non couverte de leur commande de sperme de donneur. Les coûts restants approuvés sont facturés directement à la RAMQ, simplifiant ainsi le processus pour les patients recevant un traitement au Québec.
Xytex propose une sélection de donneurs disponibles à l’achat direct sur le site web de Can-Am, rendant ces commandes immédiatement admissibles à la couverture RAMQ. Cette option offre une voie simplifiée pour le remboursement aux personnes participant au programme.
Les patients peuvent également choisir un donneur dans l’ensemble de la base de données de donneurs Xytex ; toutefois, ces commandes sont considérées comme payées par le patient lui-même et ne sont pas traitées directement via la RAMQ. Dans ces cas, il revient au patient de vérifier son admissibilité et de soumettre les demandes de remboursement par les voies appropriées auprès de la RAMQ.
Pour toute question concernant l’option la mieux adaptée à votre situation, nous vous recommandons de consulter votre clinique de fertilité ou de contacter Can-Am pour obtenir de l’aide.
Pour passer une commande auprès de la RAMQ :
1. Sélectionnez votre donneur : Sélectionnez votre donneur par l'une des deux options :
- Sur le site Web de Xytex, vous pouvez filtrer votre recherche de donneurs par les donneurs de la RAMQ. Choisissez votre donneur puis cliquez sur le bouton "Commander avec la RAMQ" ; vous serez alors dirigé vers le site de Can-Am.
- Sur le site de Can-Am, ajoutez le donneur Xytex que vous avez choisi à votre panier.
2. Sélectionnez "RAMQ" comme méthode de paiement : Choisissez "RAMQ" au moment de passer à la caisse. Vous ne serez pas facturé à l'avance ; la RAMQ s'occupera de la portion admissible des coûts.
3. Détails importants : Un seul appareil par cycle est couvert, avec un maximum de six cycles.
Les résidents du Québec admissibles au programme de procréation médicalement assistée (PMA) de la RAMQ peuvent acheter du sperme de donneur à partir d’une liste restreinte de donneurs couverts par la RAMQ, disponible via Can-Am. Toutefois, les participants ont également la flexibilité de choisir un donneur parmi le vaste catalogue complet de Xytex sur www.xytex.com, s’ils souhaitent explorer d’autres options.
Si vous choisissez un donneur directement auprès de Xytex, veuillez prendre en compte les points suivants :
Xytex est une entreprise basée aux États-Unis, et toutes les commandes sont traitées en dollars américains (USD).
Vous serez considéré comme un client « auto-payeur » et devrez payer l’intégralité des frais des paillettes de sperme et de l’expédition au moment de l’achat.
Xytex fournira une facture officielle comme preuve d’achat, que vous pourrez utiliser pour faire une demande de remboursement.
Selon la politique de votre clinique, vous pourriez être remboursé soit par votre clinique de fertilité, soit directement par la RAMQ.
Il revient au client de vérifier son admissibilité au remboursement par la RAMQ et d’entamer lui-même les démarches de remboursement auprès de sa clinique ou du gouvernement. Toutes les cliniques ne participent pas à la coordination directe avec la RAMQ pour les commandes de donneurs tiers, il est donc essentiel de parler à votre clinique avant de passer commande.
Parmi les cliniques qui soutiennent actuellement ce processus, on retrouve :
Fertilité Montréal
Fertilité Ovo
Fertilité McGill
La participation des cliniques peut varier — veuillez confirmer avec votre fournisseur de soins pour obtenir l’information la plus précise et à jour.
Si vous avez besoin d’aide pour comprendre le processus de commande ou les exigences liées au remboursement, notre équipe des relations clients se fera un plaisir de vous assister.
Can-Am fournit les factures et les reçus nécessaires au moment de la commande. Ces documents peuvent être soumis pour toute vérification supplémentaire ou si la RAMQ demande plus d'information. Votre clinique peut également fournir un certificat d'admissibilité si nécessaire.
Pour obtenir de l'aide sur le processus de commande de la RAMQ, veuillez communiquer avec l'équipe de soutien à la clientèle de Can-Am ou avec le bureau de facturation de votre clinique. L'équipe des relations avec la clientèle de Xytex est également disponible pour répondre aux questions.
Oui, le programme de procréation médicalement assistée (PMA) de la RAMQ prévoit des limites spécifiques quant au nombre d’unités de sperme de donneur couvertes par patient. Ces limites visent à garantir un accès équitable aux traitements de fertilité tout en gérant de manière responsable les ressources du système de santé.
Le nombre exact d'échantillons couvertes peut varier selon les circonstances individuelles, notamment le type de traitement (par exemple, insémination intra-utérine ou fécondation in vitro), l’admissibilité du patient et les recommandations cliniques. Il est important de consulter votre spécialiste en fertilité ou votre clinique pour comprendre les modalités de couverture applicables à votre plan de traitement.
Pour des informations précises et personnalisées, veuillez vous référer aux directives de la RAMQ ou consulter directement votre professionnel de santé.
Une fois que vous avez sélectionné la RAMQ comme mode de paiement et que votre commande a été traitée dans le cadre du programme, les annulations ou modifications peuvent être limitées en raison des procédures administratives et de facturation impliquées. Étant donné que les commandes sont coordonnées directement avec votre clinique de fertilité et le système de la RAMQ, toute modification pourrait affecter l’admissibilité à la couverture et la facturation.
Si vous devez annuler ou modifier votre commande, veuillez contacter votre clinique de fertilité dès que possible. Elle collaborera avec le distributeur et la RAMQ pour déterminer si des ajustements peuvent être effectués.
Il est recommandé de bien vérifier les détails de votre commande et de confirmer avec votre clinique avant de finaliser votre achat en utilisant la couverture RAMQ.