OUR QUALIFICATIONS
Since 1975, we have provided guidance and expertise for those ready to fulfill their dreams of starting or growing a family. We let you choose from the industry’s most selective, most tested, and most successful donors.
Selective
Our donors are chosen with you and your future family in mind. All donors entering the program after 2017 must pass a rigorous screening process, which includes personality and behavioral evaluations.
Tested
We evaluate all donors using comprehensive carrier testing covering hundreds of genetic conditions. Testing panels are regularly updated to reflect the latest advancements in genetics.
Successful
Xytex prepares all vials with 0.5 milliliters of specimen, prepared to contain a specific number of motile sperm cells. MOT refers to the total number of motile sperm cells in 1 milliliter of sample. So the minimum number of motile sperm in each vial is one half of the MOT number.
Here are two examples:
An unwashed vial (25MOT) that is loaded with 0.5 milliliters of sample will carry a minimum of 12.5 million motile sperm.
A washed vial (20MOT) that is loaded with 0.5 milliliters of sample will carry a minimum of 10.0 million motile sperm.
To view a current list of licenses, registrations and certifications, please visit our Licenses page.
We also adhere to the regulations regarding importation of products by the governments of Switzerland, Australia, and the United Kingdom (HFEA), as well as Health Canada, a federal governing board of Canada.
Your fertility journey is a very personal matter, and we respect your privacy. The information you share with us is held in strictest confidence, unavailable to anyone, other than your health care provider, without your prior permission. Your information will not be given or sold to any other company or institution. Likewise, we respect donor privacy and only release information about our donors with the donor's prior written consent. Encryption of a client's financial information is provided through Paya and Merchant Lynx.
OUR DONORS
At Xytex, we recognize how important it is for families to connect - and we’re always looking for ways to make those connections stronger. Your feedback on Famlink showed us new opportunities to better serve you, and we appreciate your support throughout this process.
We’re excited to share that we’re developing a new system: a secure database that puts you in control of how you connect with others. By allowing you to share your own contact information and choose the platforms you prefer, we’re taking moderation out of the equation - so your connections are more private and more organic.
Our goal is to have this resource ready by Summer 2025. As an employee-owned company, we believe in offering personalized experiences and genuine support to every individual on a family-building journey. Because of that, this initiative is a top priority, and we’re dedicating the time and care necessary to ensure it’s both secure and user-friendly.
Thank you for being part of the Xytex family. We look forward to providing this new platform that helps you stay connected in the ways that matter most.
A retired donor is no longer actively donating. This category of donors may or may not have remaining inventory available for purchase.
Selecting a sperm donor is a personal and sometimes complex decision, and we're here to support you throughout the process. Xytex strongly recommends consulting with your healthcare provider and a genetic counselor for individualized medical guidance as you make your selection.
To begin, create an account to access detailed donor profiles and additional selection tools. We suggest starting by identifying your key priorities—such as genetic screening, physical traits, education, or medical history—and using our search filters to help narrow your options.
If you have questions or would like more personalized support, we offer complimentary Zoom consultations with a member of our team.
Once you have selected your donor, you can place an order online or call us at 706-733-0130. Our team will be happy to guide you through the ordering process.
As of January 2019, Xytex no longer accepts Anonymous Donors into the donor program.
Clients who have reported births from an anonymous donor may still purchase vials from the remaining inventory, subject to availability.We understand the importance of a child having the opportunity to learn more about their sperm donor. That’s why Xytex offers Identity Disclosure Donors—donors who have agreed to allow their identifying information (such as full name and last known contact details) to be shared with the donor-conceived individual once they turn 18.
To be eligible to receive this information, the child’s mother—the recipient of the donor sperm—must sign the Identity Disclosure Agreement and report the child’s birth to Xytex. These steps allow us to verify the child's eligibility and ensure accurate records are maintained for future disclosure.
While a donor’s participation in the Identity Disclosure Program reflects a willingness for transparency, it does not guarantee any form of contact or relationship. It simply permits the release of identifying information when the child becomes of age and requests it.
Our online donor database is regularly updated to reflect changes in donor status and inventory. While we aim to show donors with current availability, inventory levels can fluctuate due to ongoing demand.
If a donor you are interested in is currently unavailable, we encourage you to check back regularly, as inventory may be replenished. New donors are added frequently, providing a wide range of options to support your family-building journey.
In some cases, retired donors may still have limited inventory available. If so, this will be reflected on their profile.
Please note that Sibling-Only Donors—those reserved for families who have previously reported a birth—require a documented birth on file before additional vials can be purchased.
We require both child and adult photos for our donors. However, you may come across a few donors who joined the program before this requirement was implemented, thus only childhood photos may be available.
Genetic factors can be an important part of the donor selection process. For more information about genetics and genetic testing, please view our Genetics FAQ.
At Xytex, we take donor usage limits seriously to ensure ethical distribution and safeguard the well-being of donor-conceived individuals. Each donor's availability is limited based on both the length of time he remains in the program and the number and location of reported births.
We follow the recommendations of the American Society for Reproductive Medicine (ASRM). This guideline reflects Xytex’s commitment to supporting the long-term interests of donor-conceived people by helping to limit the number of families per donor. It aligns with industry standards and is part of our effort to promote responsible donor use across regions.
To comply with these standards, we:
monitor the geographic distribution of donor vials.
- ask clients to report births so we can keep accurate records. Based on the factors listed above, access to a donor may be restricted for creating additional siblings.
Building a family looks different for everyone. Our donor options are designed to reflect your unique path and support the choices that matter most to you.
Exclusive Donors
These donors are available for purchase by a single Xytex family.XYlimited Donors
With limited inventory and a set purchase requirement, these donors are available to fewer families.
Whether you're exploring donor options, planning for future siblings, or navigating next steps—our team is here to offer guidance, clarity, and support tailored to your family-building goals.
Yes, reported pregnancies are listed on each donor’s profile. However, Xytex does not provide pregnancy success rates, as many factors—such as insemination timing, method, thawing process, and recipient fertility—can affect outcomes and are beyond our control.
A donor without reported pregnancies is not necessarily infertile. This may simply indicate they are new to the program. All Xytex donors undergo rigorous screening, including laboratory testing and physical exams, and are regularly monitored to ensure the highest quality standards throughout their participation.
Colorado's recently adopted Donor-Conceived Persons Protection Act makes several important changes starting January 1, 2025 (enforcement begins July 1, 2025). For full details on how the rules will be implemented, see Colorado’s official regulations here:https://cdphe.colorado.gov/gamete-bank/rules-regulations
Xytex is in the process of obtaining a permit to ship to Colorado under the new legislation.
In the interim, Xytex may continue to ship to Colorado under the following circumstances:
Sperm collected prior to January 1, 2025 may still be provided to recipients in Colorado.
If you have any more questions concerning Colorado compiance for a specific donor, please call our Client Relations team at 706.733.0130. We are happy to assist you.
OUR QUALITY STANDARDS
The health and medical history of our donors are extremely important. While each donor is different, they all go through the same thorough screening process.
- Each candidate completes a medical history questionnaire (MHQ).
He will answer detailed questions about his health status and provide family medical history. - Each candidate provides a semen sample for analysis.
We assess sperm count, volume, motility and additional factors to determine quality of the donation. - Each candidate is appropriately vetted.
A Donor Coordinator interviews the candidate about his personal and family medical history. During this step, the donor also undergoes a background check and education verification process.* - Each candidate undergoes psychological assessment.
This process includes a complete behavioral analysis, psychosocial, and risk assessment completed by a clinical social worker and a licensed clinical psychologist.* - Each candidate undergoes laboratory testing and a physical exam.
Following the semen sample evaluation, blood and urine samples are taken during pre-screening and are periodically screened throughout continued sperm donation.
With active participation in the program, donors undergo a physical exam and blood draw every six months. Contingent on inventory demand, some donors may be screened more frequently. A donor who remains in good health can continue to participate in our program until he reaches his 41st birthday, or until he reaches our limit of reported family units.
*Donors entering the program in 2017 or later have undergone background checks and psych-social evaluations.
- Each candidate completes a medical history questionnaire (MHQ).
While Xytex may, from time to time, get and share updated clinically significant medical and/or genetic information with Client and Client's Healthcare Provider, it is up to Client, before using any Specimens obtained from Xytex, to contact: (I) Client's Healthcare Provider; and (II) Xytex, for any such updated information of which Xytex may have become aware. Client acknowledges and agrees that under some circumstances, Xytex may have shared such updated information only with Client's Healthcare Provider, in which event, whether or not Client would be informed of such updated information would be entirely dependent upon Client's contacting Client's Healthcare Provider and receiving such updated information from Client's Healthcare Provider.
Although Xytex is not obligated hereunder (or otherwise) to disclose or share with Client or Client's Healthcare Provider any updated clinically significant medical and/or genetic information, in the event Xytex does share any such updated information, an experienced genetics counselor should be consulted to advise Client as to its potential significance. Xytex is not a medical provider and cannot provide medical advice.
We conduct laboratory testing and physical exams on all donors to assess their overall health. Additionally, we conduct genetic testing on all donors to help you confidently choose your family.
Screening looks for evidence of, the most common symptoms of a particular infection. Testing involves applying laboratory methods to biological specimens, such as blood, urine, or semen, to determine the presence of a specific infectious agent, such as a bacteria or a virus.
Fresh semen is unsuitable for sperm banking since it begins to lose fertility within an hour or two of ejaculation. Because a sperm bank is expected to have semen available from a diverse set of donors with different physical characteristics and different talents, it is necessary to store sperm from the time of collection until it is needed. We accomplish this through a unique freezing process called cryopreservation, which gives semen an extended survival period. It is the only way sperm can be shipped from our bank to a client, no matter where they live. Once thawed, cryopreserved sperm can be as fertile as fresh sperm.
Sperm is also cryopreserved to allow a quarantine period and retesting of various diseases, such as HIV which causes the potentially-lethal AIDS infection. Cryopreserved sperm must be stored for a minimum of six (6) months before repeat testing of the donor. Repeat testing includes HIV 1/2 + O antibodies, HTLV I & II antibodies, hepatitis B surface antigen, hepatitis B core antibody, hepatitis C, HIV/HCV/HBV NAT, TP-PA, and cytomegalovirus (CMV) antibodies. Donors are also tested for chlamydia and gonorrhea.
Scientists believe the potential for long-term storage is indefinite, assuming adequate maintenance of low temperatures.
Before 10/01/2017, Xytex semen was cryopreserved in sterile-filtered Test Yolk Buffer (Irvine Scientific, Santa Ana, CA), composed of buffers (TES and Tris), sodium citrate, fructose, gentamicin sulfate, glycerol, and heat-activated egg yolk from specific pathogen-free laying flocks. Detailed product information can be found at https://www.irvinesci.com/assisted-reproductive-technology/sperm-preparation-media/freezing-medium-tyb-with-glycerol-gentamicin.html
Between 10/01/2017 and 05/01/2018; Units were cryopreserved with Sperm Maintenance Medium (Irvine Scientific, Santa Ana, CA). The medium does NOT contain egg yolk or antibiotics. It does contain HEPES and human serum albumin.
As of 05/01/2018, donor sperm is cryopreserved in ArcticTM Sperm Cryopreservation Medium (Irvine Scientific, Santa Ana, CA). The medium does NOT contain egg yolk or antibiotics. It does contain HEPES, MOPS, and human serum albumin. Detailed product information can be found at http://www.irvinesci.com/products/90170-arctic-sperm-cryopreservation-medium.
Comparing human births resulting from thawed semen to non-assisted pregnancies shows no difference in the rate of birth defects or miscarriage.
Cytomegalovirus (CMV) is a common virus that infects approximately 70% of persons in the United States by age 45 and more than 90% by age 80.? In most cases, initial infection results in no symptoms, though some people experience a mild illness, similar to the flu or mononucleosis.? After the initial infection, the virus remains dormant (latent) in the body.? Reactivation of infection typically occurs in persons with compromised immune systems.
If a woman who has never been exposed to CMV becomes infected with the virus during pregnancy, there is a 40% risk of fetal infection.? In contrast, the risk of fetal infection is only 1% in women with previous CMV exposure.? Fetal infection with CMV can result in miscarriage and serious developmental defects in approximately 10% of cases where primary infection occurs during the first 14 weeks of pregnancy.
Xytex tests all donors for CMV and includes each donor’s status in his profile. CMV status is based on testing blood for antibodies (IgG and IgM) to the virus. CMV-negative donors have no IgG or IgM antibodies. CMV-positive donors have IgG antibodies that persist for life, but the pattern of antibodies determines whether infection is latent (inactive) or active. Xytex does not accept sperm from donors whose test results suggest active or recent CMV infection (IgM-positive). Sperm from donors with latent infection (IgG-positive, IgM-negative) is considered to be safe.
Knowing your CMV status can help navigate your fertility journey. While it might seem reasonable to accept only CMV-negative donors, such a restriction would reduce the donor pool by more than half, leaving women with fewer choices. Xytex encourages clients to discuss CMV with their healthcare providers, who may recommend CMV testing. Providers often recommend that CMV-negative patients use sperm from CMV-negative donors.
OUR PROCESS
Xytex relies on clients to report all births resulting from donor sperm use. Births should be submitted through your Xytex website account portal.
Timely birth reporting is essential to:
Maintain accurate tracking of family units per donor
Ensure continued access to the donor for clients building genetically related families, pending vial availability
Allow ongoing access to the donor’s profile and pertinent donor updates, such as medical or genetic information
Uphold the integrity, safety, and compliance of our donor program
Your cooperation helps protect donor-conceived children, supports responsible family planning, and strengthens the transparency and long-term success of the donor community.
We have made it easier than ever to manage your vial shipments directly from your Xytex Client Portal.
To get started:
1. Log in to your Client Portal at xytex.com.
2. Navigate to the My Orders tab.
3. There, you will see a list of your stored vials.
4. Simply select the vials you wish to ship, choose the destination, and confirm the shipment details.
5. Submit your request, and our team will take care of the rest!
This feature allows you to schedule shipments at your convenience—anytime, from anywhere.
Need help or have questions?
Our Customer Relations Team is ready to assist you.
Email: info@xytex.com
Call: 706-733-0130 or 1-800-277-3210We are here to make the process smooth and stress-free.
ART vials are often used in IVF (in vitro fertilization) – sperm and egg are combined in a petri dish for fertilization.
An unwashed vial is frozen in its most natural state, allowing sperm to remain in its natural seminal fluid. Unwashed vials are used for ICI (intracervical insemination) – sperm enters through the cervix, much like in sexual intercourse.
A washed vial separates the sperm from its natural seminal fluid and is frozen in preserving fluid. Washed vials are used for IUI (intrauterine insemination). While we offer washed vials prepared for IUI treatment, we also offer unwashed vials, which are suitable for IUI, but require washing by the inseminating clinic.
*Please consult your Clinic for the appropriate vial type for your procedure.
Please also see this helpful video!
We are thrilled to partner with CapexMD, the leader in providing patient financing for fertility treatments. With an easy process and competitive rates, finances don’t have to be an obstacle to achieving your dream of a family.
Some of the many advances of financing through CapexMD:
- Specialists in Fertility Financing
- Competitive Rates
- Easy & Secure Online Application
- Quick Approval (within 24 hours)
- No Annual Fees
- Flexible Terms
If you’re nearing treatment and purchasing multiple vials or planning for the future, your purchase can be financed with a CapexMD loan*.
*minimum loan amount is $3000
First, create an account and browse through our catalog of sperm donors. With our interactive features, you can view child and adult photos and gain insight from donors’ personal essays. When narrowing your search, we urge you to choose several of your top picks and rank them in order of preference.
When you’re ready, give us a call, and we will check availability of your top picks, making every effort to secure your first-choice. However, due to normal fluctuations in our inventory, this is not always possible. If you'd like to use the same donor for future children, we recommend buying additional vials during your initial purchase.
We also recommend consulting your healthcare provider when choosing a donor. They will be able to help you navigate things like expected day of ovulation and the number and type of vials needed for your procedure. We strongly recommend planning to have your vials delivered a day or two prior to ovulation.
Xytex is unable to facilitate the transfer of ownership of your vials to a third party. Xytex has a strict policy prohibiting this type of transfer, as Xytex cannot guarantee the quality or integrity of the vials once shipped from our facility or another designated storage facility. In furtherance of this policy, clients complete a service agreement that states they are purchasing the vials for their own use or use by a partner and are not permitted to transfer them to any other entities. By following these policies, Xytex can maintain vial safety, compliance, international family limits and properly observe applicable laws.
Xytex ships donor specimens directly to your home residence, offering greater flexibility and added privacy for your fertility journey. However, it’s important to be aware that state laws vary regarding at-home insemination and legal parentage.
Key Legal Considerations:
Some states require insemination to be performed only by a licensed physician. At-home insemination may not be legally recognized in those states.
In certain states, a husband must provide written consent prior to insemination for his name to appear on the birth certificate.
Other states may require proof of insemination with anonymous donor sperm for the recipient’s partner to be listed as a legal parent.
We strongly recommend consulting with your healthcare provider or legal advisor to ensure that at-home insemination is permitted and properly documented under your state’s regulations.
Special Note for New York Residents:
Home delivery is available in New York only with additional documentation completed by your licensed healthcare provider. Please review your state’s requirements before proceeding with your order.
Home Delivery Policy:
Orders can be placed online and shipped directly to your residence for convenience and privacy.
A non-refundable tank deposit of $875 is required for all home deliveries.
An unwashed vial is frozen in its most natural state, allowing sperm to remain in its natural seminal fluid. A washed vial separates the sperm from its natural seminal fluid and is frozen in a preserving fluid. Removing seminal fluid helps minimize cramping that can result from prostaglandins, the hormones that cause the uterus to contract.
Washed vials should be used in IUI procedures, as unwashed vials injected into the uterus can cause cramping. In addition to being extremely painful, it will most likely result in the loss of injected sperm. Both washed and unwashed samples can be used for ICI or intracervical inseminations. ART samples are processed in exactly the same way, with the only difference being the minimum number of motile cells.
Both ICI and IUI vials are suitable for at-home vaginal insemination, and no additional preparation is needed. There are different volumes and cell numbers associated with each vial type, but these are in reference to the pre-purchase processing that occurs in our laboratory. Please see our Quality Commitment (https://www.xytex.com/about-xytex/quality-commitment/) for additional helpful information. *IVF vials are not intended nor recommended for at-home vaginal inseminations.
While it may not take 4 vials, national averages range from 3-4 insemination cycles per successful pregnancy. The only way to guarantee your donor will be available is to plan ahead. With the purchase of 4-7 vials, you will receive 1 year of free storage and 1-year buyback at 50% of the original purchase cost (when stored at Xytex). When a donor sells out, it saddens us to have to break the news to a client who hopes for a brother or sister for their child. If you intend to use the same Donor throughout your complete insemination process or desire more than one child from the same Donor, it's critical that you buy more now and store them at Xytex for future use. Enjoy 3 YEARS of FREE storage with the purchase of 8 or more vials. Once pregnant, you can extend your storage term for future siblings (just in case) or take advantage of the 50% Vial Buyback Program.
You or your health care provider can place an order by calling our Client Relations team at 706-733-0130. We also offer online ordering for some donors*. If online ordering is an option, it will appear at the bottom of a donor's profile. Shipments will only be sent once your health care provider signs our Supply Agreement form. *online ordering applies to US sales only
Please see the Xytex Forms page, specifically:
Donor Sperm Services Agreement
Shipping AgreementSee Also:
How To Sign My DocusignXytex understands that shipping to an alternate location may be desirable in certain circumstances. You may download the “Homeship” form (“Authorization for Release of Direct to Clients Shipments”) from the Xytex Forms page. Xytex recommends that clients and physicians become familiar with the laws governing artificial insemination in their state of residence.
We accept wire transfers and all major credit cards.
Your order is shipped FedEx Next Day or 2nd Day, depending on what option you select. Please note: we only ship orders Monday through Friday from 9 am to 5 pm EST, and our Client Servies Team is only available during these business hours.
*Holiday Shipping: Please Plan Ahead! Due to heavy shipping volume, COVID related business closures, and winter weather disruptions, your order may be delayed. Visit the FedEx website to see how your shipment may be affected: https://www.fedex.com/en-us/service-alerts.html
We recommend that you request your shipment to arrive at least three days prior to insemination. FedEx Priority Overnight shipping will arrive the next business day by 12 pm. FedEx Two-Day shipping will arrive the second business day by 12 pm.*
*Overnight shipping may be affected by weather or Federal Express equipment failure. To help ensure your vials arrive in time, please plan for your shipment to be delivered 3 days prior to your insemination
*Holiday Shipping: Please Plan Ahead! Due to heavy shipping volume, COVID related business closures, and winter weather disruptions, your order may be delayed. Visit the FedEx website to see how your shipment may be affected: https://www.fedex.com/en-us/service-alerts.html
Each vial arrives frozen in a screw-cap unit that is clipped to a metal support rod (a cane) to aid removal from the refrigerated tank (dry shipper). The tank is cooled with liquid nitrogen trapped in spongy material to prevent spillage, hence the name dry shipper. Each tank is shipped in a cardboard box which includes a packing slip with the Summary of Records (required by the FDA), thawing instructions, a letter from our Medical Director, a return shipping label and applicable invoices.
Please Plan Ahead! Due to heavy shipping volume and COVID related business closures, your order may be delayed. Visit the FedEx website to see how your shipment may be affected: https://www.fedex.com/en-us/service-alerts.html
Once you have selected the perfect donor, simply place your order. The vials will be shipped to your healthcare provider’s office in a sealed, refrigerated tank that will preserve your sample(s) for up to seven days. Two-day delivery options are available.
*Holiday Shipping: Please Plan Ahead! Due to heavy shipping volume, COVID related business closures, and winter weather disruptions, your order may be delayed. Visit the FedEx website to see how your shipment may be affected: https://www.fedex.com/en-us/service-alerts.html
If your vials are being stored at Xytex or CANAM:
With a purchase of 6 or more vials, the 1st swap is complimentary. Each additional swap is $895.
With a purchase of 5 vials or less, there is a first swap fee of $150. Each additional swap is $895.
Once vials have left Xytex or an approved storage facility, they are ineligible for returns or exchanges.
The easiest way to ensure access to the same donor for future inseminations is to buy additional vials during your initial purchase. You can request to store them at our facility until needed for treatment.
We have top-notch donors, and they sell out quickly! When a donor sells out, it saddens us to have to break the news to a client who hopes for a brother or sister for their child. If you intend to use the same Donor throughout your complete insemination process or desire more than one child from the same Donor, it's critical that you buy more now and store at Xytex for future use.
Receive 1 Year of FREE storage for 4 or more vials, simply for planning ahead.
Enjoy 3 YEARS of FREE storage with the purchase of 8 or more vials.
Once pregnant, you can extend your storage term for future siblings (just in case) or take advantage of the 50% Vial Buyback Program.
Sign in to “My Account” and visit the Birth Report tab.
Since 1975, we have provided the expertise your patients need to fulfill their dreams of starting or growing a family. We let your patients choose from the industry’s most selective, most tested, and most successful donors.
Selective
Our donors are chosen with your patient and her future family in mind. Since January 2017, all donors have endured a rigorous screening process, which includes personality and behavioral evaluations.
Tested
We evaluate all donors for hereditary conditions using an extensive medical history questionnaire and carrier testing for genetic conditions.
Successful
Our donors have a minimum 12.5 million motile sperm per 0.5 mL in unwashed vials and 10.0 mL motile sperm per 0.5 mL in washed vials.
To view a current list of licenses, registrations and certifications, please visit our Licenses page.
We also adhere to the regulations regarding importation of products by the governments of Switzerland, Australia, and the United Kingdom (HFEA), as well as Health Canada, a federal governing board of Canada.
We comply with expectations published by the FDA in regards to Human Cells, Tissues, and Cellular and Tissue-Based Products (21 CFR Part 1271).
While we are willing to send donor specimens to any address specified on a client’s order form, regardless of the shipment's destination, we require clients to be under the care of a healthcare provider.
You must sign an Authorization to Ship to an Alternate Location form (located on the Xytex Forms page) before having any specimens shipped directly to the patient.
You want someone to care for your patients with compassion, support, and expert guidance. We offer an empowering experience, providing them with the best chance of conceiving a healthy baby. With Xytex, you get a partnership based on shared goals. It’s more than a transaction.
To start a partnership and establish a Physician Account, we ask that you complete a Supply Agreement form (located on the Xytex Forms page) which will allow you to designate the party responsible for paying for services – the clinic or the patient. Orders may be placed by the health care provider or clinic employees, but all orders will be confimed with the clinic prior to shipment.
We conduct laboratory testing and physical exams on all of our donors to assess their overall health. Additionally, we conduct genetic testing on all of our donors to help your patients confidently choose their family. An extensive list of donor testing can be viewed here.
During active participation in the program—those who regularly provide samples of blood, semen and urine—undergo a physical exam and a blood draw every six months.
Our screening and testing complies with government licensing agencies and is consistent with guidelines established by the FDA. Additionally, the United States Food and Drug Administration has regulations (21 CFR Part 1271) for screening and testing for specific sexually transmitted infections (STI). In addition to STIs, the state of New York and several organizations expect screening and testing for a few genetic conditions.
CMV is a common virus that infects 70% of persons in the United States by the age of 45 and more than 90% by the age of 80. In most cases, initial infection results in no symptoms, though some people experience a mild illness, similar to the flu or mononucleosis.? After the initial infection, the virus remains dormant (latent) in the body.? Reactivation of infection typically occurs in persons with compromised immune systems.
If a woman who has never been exposed to CMV becomes infected with the virus during pregnancy, there is a 40% risk of fetal infection.? In contrast, the risk of fetal infection is only 1% in women with previous CMV exposure. Fetal infection with CMV can result in miscarriage and serious developmental defects in 10% of cases where primary infection occurs during the first 14 weeks of pregnancy.
Approximately 1% of newborns in the United States are infected with CMV.? This is most often a result of primary maternal infection or maternal reactivation.?Congenital CMV infection may result in significant psychomotor, hearing, ocular, or dental abnormalities.
Tests for CMV antibodies can distinguish between current (recent) infection and latent infection. Individuals with current or recent infection have IgM antibodies in their blood. After an acute infection has resolved, IgM antibodies disappear; however, IgG antibodies persist indefinitely.
Xytex tests donors for CMV antibodies at least every six (6) months. Approximately 60% of Xytex donors are IgG-positive. Sperm from IgM-positive donors is not accepted.
We encourage you to discuss CMV with your patients. You are best suited and qualified to explain this issue and provide guidance. If you recommend using a CMV-negative donor, please ask your patient to call Xytex at 1-706-733-0130 or email info@xytex.com to confirm the donor's CMV status before placing an order.
Each vial arrives frozen in a screw-cap unit clipped to a metal support rod (a cane) to aid removal from the refrigerated tank (dry shipper). The tank is cooled with liquid nitrogen trapped in spongy material to prevent spillage, hence the name dry shipper. Each tank is shipped in a cardboard box. The shipment includes a packing slip with the Summary of Records (required by the FDA), thawing instructions, a letter from our Medical Director, a return shipping label, and applicable invoices.
We arrange for pickup of the tank on the eighth day after shipping from our facility. The tank should be sealed in the original shipping box and the return label applied to the outside surface of the box.
A daily rental fee of $40 applies to any tank kept beyond the pickup date. Given advance notice, we will work with your office to alleviate extenuating circumstances. We hold the health care provider and/or clinic financially responsible, not the patient, for any unreturned or damaged tank.
While Xytex may from time to time share updated clinically significant medical and/or genetic information with Client and Client's Healthcare Provider, it is up to Client, prior to using any Specimens obtained from Xytex, to contact: (I) Client's Healthcare Provider; and (II) Xytex, for any such updated information of which Xytex may become aware.
It is up to the Client to check the Donor's profile on the website for updated medical information. Client acknowledges and agrees that under some circumstances, Xytex may have shared such updated information only wtih Client's Healthcare Provider, in which event, whether or not Client would be informed of such updated information would be entirely dependent upon Client's contacting Client's Healthcase Provider and receiving such updated information.
Although Xytex is not obligated hereunder (or otherwise) to disclose or share with Client or Client's Healthcase Provider any updated clinically significant medical and/or genetic information, in the event Xytex does share any such updated information, an experienced genetics counselor should be consulted to advise Client as to its potential significance. Xytex is not a medical provider, and cannot provide medical advice.
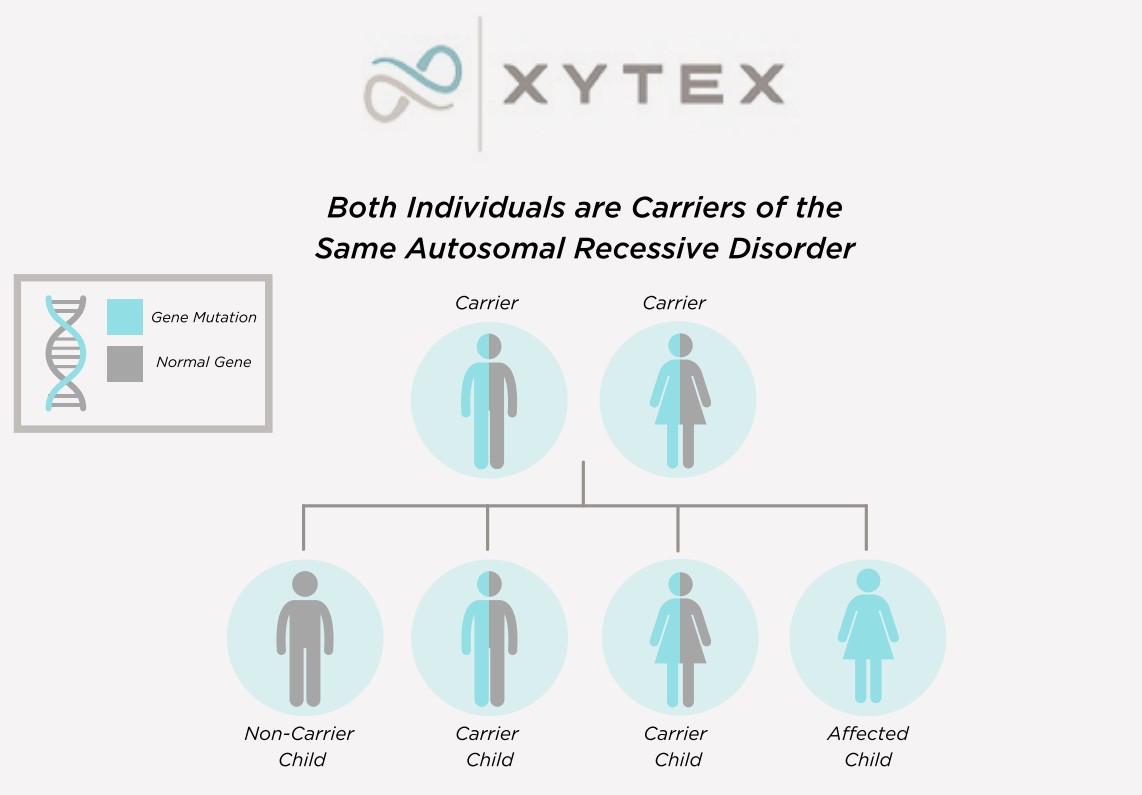
Many genetic conditions have an autosomal recessive pattern of inheritance. To be autosomal recessive, both copies of the gene must be mutated (altered) in order to cause the condition. If only one copy is mutated, a person is considered to be a carrier of the condition but does not have the actual condition.
Help, My Sperm Donor Is A Carrier Of A Genetic Disease
Video by Jamie K. Dokson, ScM, LCGC(Licensed Certified Genetic Counselor)We perform carrier testing on our donors to help reduce the risk of offspring inheriting common autosomal recessive conditions. Each donor’s profile includes a list of his genetic conditions, along with the results for which he was tested.*
*Genetic testing has changed over time, and some donors may have been tested for fewer conditions and with different methodologies. Incoming Xytex donors now undergo comprehensive carrier screening, covering hundreds of genes, including those recommended by the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics (ACMG).
Negative genetic testing only reduces the possibility that a donor is a carrier of a particular condition.
While we do accept donors who are found to be carriers of genetic conditions, to purchase sperm of a donor known to carry a genetic condition, a client must sign a waiver stating her understanding of the associated risks.
Additionally, if a female is a known carrier of a specific genetic condition, we will provide a copy of the donor’s test reports so that you can review this information with your health care provider and genetic counselor and make the most educated decision with regards to the health of your potential offspring.
It is estimated that 1 in 100 children are born with a genetic condition. The incidence of genetic conditions varies among ethnic groups. For example, Sickle Cell disease is more common among persons of African ancestry than Caucasians of European descent.
Xytex continuously updates its carrier screening panels to cover hundreds of genetic conditions, increasing options and reducing genetic risks. However, if you are a carrier of a genetic condition for which a donor was not tested, we can attempt to contact the donor to request additional carrier testing. While we strive to accommodate these requests, we cannot guarantee that the donor will agree to further testing. Please email info@xytex.com or call 1-706-733-0130 for additional information.
Sequencing of a gene involves reading a gene from beginning to end and comparing the code to a database of known normal and abnormal (pathologic) mutations. This method of genetic testing is more extensive. A negative result from sequencing reduces the likelihood of carrier status more than targeted mutation analysis, which only detects a few of the most common mutations of a particular gene. Genetic testing has changed over time, and some donors may have been tested for fewer conditions and with different methodologies. Men who joined our donor program since January 2019 have undergone comprehensive carrier testing of hundreds of conditions using next-generation sequencing, providing detailed genetic insights.
To initiate a home shipment, Xytex clients must complete the required Home Shipment Authorization forms and Genetic Waivers through DocuSign. These documents ensure that clients understand the process and acknowledge the associated considerations when choosing to proceed with at-home insemination.
As of our current policy, Xytex no longer requires the signature of a licensed medical professional to approve at-home inseminations for most U.S. residents. However, clients residing in the state of New York are subject to additional state regulations and must obtain written approval from their licensed physician before vials can be shipped to their home.
Please note that home shipments are available to U.S. clients only at this time. For assistance completing the necessary documentation or determining your eligibility, our Client Services Team is available to guide you through the process.
*Genetic waiver necessary only if the donor is a carrier of a genetic condition.
We have made it easier than ever to manage your vial shipments directly from your Xytex Client Portal.
To get started:
1. Log in to your Client Portal at xytex.com.
2. Navigate to the My Orders tab.
3. There, you will see a list of your stored vials.
4. Simply select the vials you wish to ship, choose the destination, and confirm the shipment details.
5. Submit your request, and our team will take care of the rest!
This feature allows you to schedule shipments at your convenience—anytime, from anywhere.
Need help or have questions?
Our Customer Relations Team is ready to assist you.
Email: info@xytex.com
Call: 706-733-0130 or 1-800-277-3210We are here to make the process smooth and stress-free.
Understanding Vial Types for At-Home Insemination
At-home vaginal insemination may be performed using either IUI or ICI vials. It is important to understand the differences:
IUI (Intrauterine Insemination) vials are washed, meaning seminal fluid and non-motile sperm have been removed in our lab. These vials are processed for direct placement into the uterus but may also be used for vaginal insemination at home if advised by your provider.
ICI (Intracervical Insemination) vials are unwashed, meaning they contain the full ejaculate, including seminal plasma. These are generally used for vaginal or cervical insemination.
Each vial type differs in volume and motile sperm count due to the lab processing involved. For this reason, we strongly recommend that you consult with your healthcare provider before purchasing, to ensure the selected vial type aligns with your health history, insemination method, and overall fertility plan. Your provider can help you make the most informed decision based on your goals and comfort with at-home procedures.
Please Note: IVF (ART) vials are intended for use in assisted reproductive technologies such as IVF and are not recommended or approved for at-home vaginal insemination.
Important Shipping Policy
Vials shipped for at-home insemination are not eligible for return, replacement, or a Quality Commitment Report. Once shipped, all sales are considered final.
Cost and Shipping Information for At-Home Insemination
When ordering vials for home insemination, clients are responsible for the cost of the vial(s) and shipping fees, which vary based on destination and delivery timeframe.
A refundable tank deposit of $875 is also required. This deposit covers the use of a liquid nitrogen tank that protects the vial(s) during transit. Once the tank is returned to Xytex in good condition, your deposit will be refunded within 8–10 business days of its arrival back at our facility.
Vials shipped in our cryogenic tanks are guaranteed to maintain quality for up to 7 days from the date of shipment. Once the tank is opened, vials must be used within 24 hours to ensure optimal viability.
If you have questions about timing or tank handling, our Client Relations team is here to help guide you through the process.
If you're not ready to use your vials immediately, Xytex offers secure long-term storage options at our FDA-registered facilities. Storing your vials with us ensures they are maintained under strict cryogenic conditions, preserving their quality until you’re ready to proceed with treatment.
You can purchase vials now to reserve inventory from your selected donor and store them at Xytex for as long as needed. We offer flexible billing options for storage—monthly, quarterly, or annually—so you can choose what best suits your needs and planning. Our Client Relations Team is here to provide personalized support, whether you need help managing your storage account, updating billing preferences, or coordinating a shipment when the time is right. We're committed to supporting you every step of the way.
This flexible option provides peace of mind and helps you plan ahead with confidence.
How long will my vials remain frozen?
Your vials will remain safely frozen in the cryogenic shipping tank for up to 7 days from the date of shipment. The tank is pre-filled with liquid nitrogen to maintain the extremely low temperatures required to preserve the quality and viability of the sperm during transport and temporary storage.
We recommend that the tank remain closed until you are ready to proceed with insemination. Once the tank is opened, the vials should be used within 24 hours to ensure the best possible outcome.
If you anticipate any delay in using your vials or have questions about timing, please contact our Client Relations team—we're happy to help you plan accordingly.
No. Vials must remain in the liquid nitrogen shipping tank until you are ready to use them. Standard household freezers do not reach the temperatures required to safely preserve sperm and can damage the vial contents. For best results, keep the tank sealed and follow usage instructions provided.
What supplies do I need for home insemination?
At-home insemination can be a simple and empowering option for many families. To help you prepare, we’ve outlined the basic supplies you’ll need for the process:
Sperm Vial
You’ll receive your selected donor sperm vial—either IUI (washed) or ICI (unwashed)—based on your treatment plan and provider recommendation. Be sure to confirm with your healthcare provider which vial type is best suited for your needs.Sterile Syringe
A sterile 1 mL syringe is included with your shipment. This is used to transfer the thawed sperm for vaginal insemination. If you prefer or require a different style of syringe, speak with your provider before your shipment date.Disposable Gloves
Using gloves can help maintain a clean and hygienic environment during the insemination process.A Comfortable, Quiet Space
You’ll want a clean and private area where you can lie down for 15 to 30 minutes after insemination to allow sperm the best chance to reach the cervix.A Timer or Clock
Keeping track of how long you rest after the insemination can help you stay relaxed and consistent with your timing.
Helpful Reminders:
Keep the shipping tank sealed until you're ready to proceed. Once opened, the vial should be used within 24 hours.
Do not attempt to store the vial in a household freezer. The vial must remain in the liquid nitrogen tank until use.
If you have any questions about the process, our Client Relations team is here to support you.
Your shipment from Xytex will contain the following items:
- Your donor sample vials.
One needle-free insemination syringe for each vial included in your order, designed for ease of use and comfort.
Comprehensive vial thaw instructions to help you properly prepare the specimen for insemination, ensuring optimal viability.
Please note that while we provide the necessary materials and instructions for insemination, it is important to follow the guidance of your healthcare provider or fertility specialist regarding your specific treatment plan and insemination timing. If you have any questions or concerns about the process, do not hesitate to reach out to your clinician for personalized support.
We understand that timing of insemination is one of the most important factors in achieving a successful pregnancy. Because each person’s cycle and fertility journey is unique, Xytex strongly encourages clients to consult with their healthcare provider or fertility specialist to determine the optimal time for insemination based on ovulation tracking and personal medical history.
For at-home insemination, your provider may recommend using ovulation predictor kits or other cycle-tracking tools to identify your most fertile window. In most cases, insemination is advised to occur within 24 hours of a positive ovulation test.
Additionally, many healthcare professionals recommend a second insemination within 24 hours of the first to increase the likelihood of conception. Be sure to discuss this option with your provider to determine what’s best for you.
If you are working with a clinic, your care team will schedule insemination (IUI or IVF) based on monitored ovulation or hormonal triggers.
At Xytex, we’re here to support you every step of the way and encourage you to reach out to our Client Relations Team for any assistance or to coordinate the timing of your shipment.
For individuals with no known fertility concerns, home insemination has an average success rate of 10–15% per cycle. This is similar to the natural conception rate through unassisted intercourse. It’s important to understand that conception is often not immediate—many individuals and couples may require multiple cycles before achieving a successful pregnancy.
Several factors can influence your chances of success with home insemination, including:
Age of the person trying to conceive
Timing of the insemination in relation to ovulation
Proper thawing/handling
Underlying medical conditions or reproductive history
While home insemination can be a convenient and accessible option for many, it’s not the right fit for everyone. That’s why Xytex strongly recommends consulting with your healthcare provider before beginning. A clinician can help assess your reproductive health, guide you on timing, and ensure that at-home insemination is a medically appropriate and safe option for your individual circumstances.