Donor Screening Process
Evaluation
The Food and Drug Administration (FDA) and other regulatory entities require a multi-step process to determine if an individual is eligible to become a sperm donor and to continue donating. The first step involves screening the donor by obtaining reviewing the donor-reported personal and family medical history. The second step involves laboratory testing for relevant infectious diseases. The third step involves a brief, targeted physical examination that focuses on clinical findings of and risk factors for infectious diseases. After the initial evaluation, these steps are repeated at least once every 6 months to ensure that the donor remains eligible to continue donating.
Although not required by the FDA, Xytex performs genetic testing of all donors, including hemoglobin analysis, carrier testing for hundreds of genetic conditions*, and chromosome analysis (karyotyping).
*Prior to 2017, carrier testing of donors included a smaller number of conditions. Donor profiles list all conditions included in carrier testing. Xytex recommends downloading the actual test reports from donor profiles for careful review, as the reports contain important detailed information.
Screening
The process of becoming a sperm donor process begins with in-depth screening. The health and medical history of our donors are of utmost importance. While each donor is different, they all go through the same thorough screening process.*
- Each candidate completes a medical history questionnaire (MHQ).
Detailed questions are asked about his health status and family medical history. - Each candidate provides a semen sample for analysis.
We assess sperm count, volume, motility, and additional factors to determine if the specimen is suitable for donation. - Each candidate is appropriately vetted.
A Donor Coordinator interviews the candidate about his personal and family medical history. During this step, the candidate also undergoes a background check and education verification. - Each candidate undergoes a psychological assessment.
This process includes a complete behavioral analysis and psychosocial and risk assessments completed by a clinical social worker and a licensed clinical psychologist. - Each candidate undergoes a brief, targeted physical examination.
*All donors entering the program after 2017
Donor privacy is very important to us. Donor and client agreements protect and release donors from any obligations or rights to a child that results from use of donor sperm. No identifying information is released to clients or offspring without the donor’s prior consent.
Questions developed by the FDA and U.S. Centers for Disease Control and Prevention (CDC) are included. Any affirmative answer to a required question disqualifies the candidate. All questionnaires are reviewed by and must be approved by our Medical Director for a candidate to continue through our screening process. FDA-mandated screening and testing also ensures our donors are in continued good health.
Please note that medical history provided by the donor is not validated by reviewing the donor’s or his family’s personal medical records.
Lab Testing
As part of our commitment to provide families with the highest quality sperm, Xytex conducts laboratory testing and physical exams on all of our donors to assess their overall health.
- Blood typing
- Human immunodeficiency virus (HIV), types 1/2/O via antibody testing
- HIV via nucleic acid testing (NAT)
- HIV p24 antigen (Ag)
- Hepatitis B virus (HBV) via core antibody (HBcAb) testing
- HBV via surface Ag (HBsAg) testing
- HBV via NAT
- Hepatitis C virus (HCV) via antibody testing
- HCV via NAT
- Human T-lymphotropic virus (HTLV), types 1/2 via antibody testing
- West Nile virus (WNV) via NAT
- Syphilis via treponemal-specific antibody testing
- Chlamydia via urine NAT
- Gonorrhea via urine NAT
- Cytomegalovirus* (CMV) via total antibody testing with reflex to IgG and IgM antibody testing for a positive result
We test all donors for Cytomegalovirus (CMV), an extremely common virus that nearly every adult has at one time or another. Although dormant after the initial infection, CMV stays in the body’s system for life. We accept samples from a donor with an IgG positive result, which indicates a non-active CMV infection. An IgM positive result indicates an active CMV infection and the donor is considered unacceptable. If you have any questions or concerns, we suggest you discuss the CMV status of a donor with your doctor prior to purchasing vials. You will note the appropriate icon beside each donor to reflect his CMV IgG status. Whether the icon indicates positive or negative does not qualify or disqualify the donor.
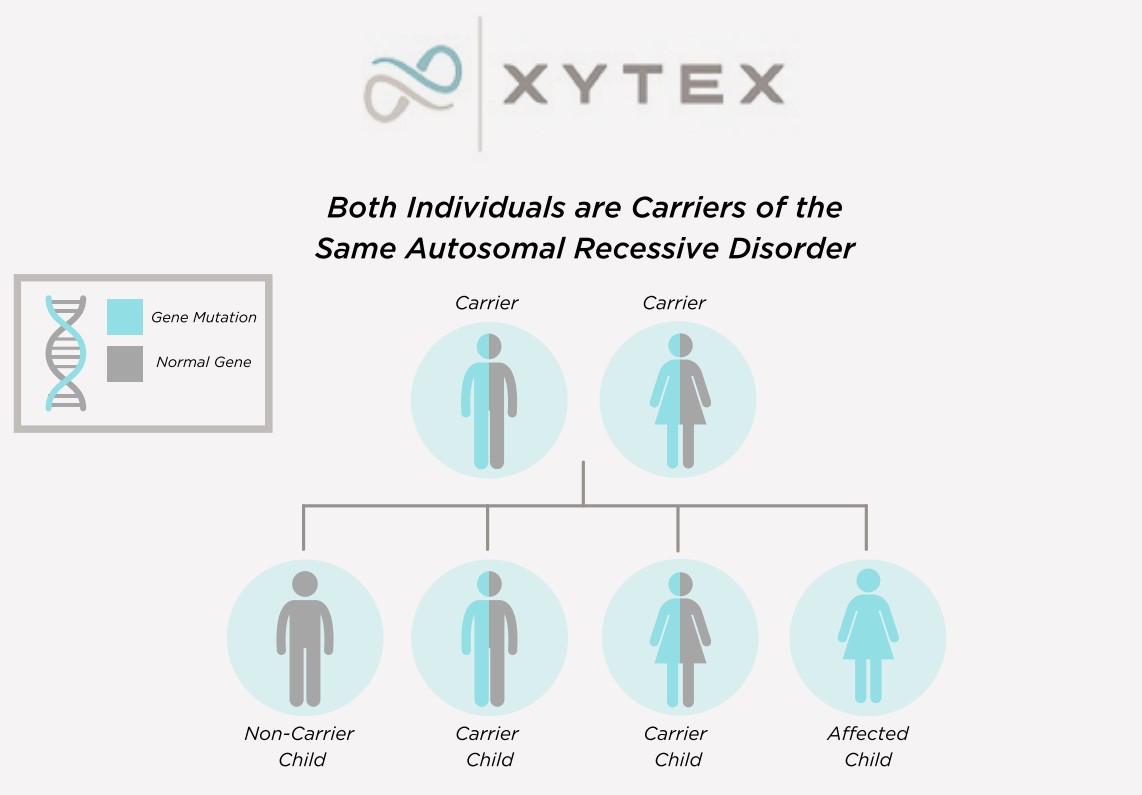
Genetic Testing
Expanded carrier testing is here!
Donors joining our program now undergo carrier testing for hundreds of inherited conditions.
Each donor’s profile includes a list of genetic conditions for which he has been tested. If there is a change in status, the donor’s profile is updated with the new information.
Please check individual profiles for a list of screened conditions specific to each donor.
Additional Resources
Help, My Sperm Donor Is A Carrier Of A Genetic Disease
Video by Jamie K. Dokson, ScM, LCGC(Licensed Certified Genetic Counselor)
xyGene Donors
Each xyGene donor undergoes expanded carrier testing of over 100 genetic conditions. *
- 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- (CYP21A2)
- Alpha-1 Antitrypsin Deficiency (SERPINA1)
- Alpha-Thalassemia (HBA1/HBA2)
- Beta-Thalassemia (HBB)
- Familial Mediterranean Fever (MEFV)
- Galactosemia (GALT)
- Krabbe Disease (GALC)
- MCAD Deficiency (ACADM)
- Phenylalanine Hydroxylase Deficiency (PAH)
- Polycystic Kidney Disease, Autosomal Recessive (PKHD1)
- Pompe Disease (GAA)
- Smith-Lemli-Opitz Syndrome (DHCR7)
- Very Long Chain Acyl-CoA Dehydrogenase Deficiency (ACADVL)
- Wilson Disease (ATP7B)
- Zellweger Spectrum Disorder, PEX1-Related (PEX1)
* Donors entering the program after January, 2019 have been screened for more than 280 carrier conditions: Sema4
Negative genetic testing only reduces the possibility that a donor is a carrier of a particular condition.
Genetic testing changes over time and some donors may have been tested for fewer conditions. Donors who joined our program prior to 2012 may not have been tested for all conditions listed above.
While we do accept donors who are found to be carriers of genetic conditions, to purchase sperm of a donor known to carry a genetic condition, a client must provide proof that she does not carry the condition and has received genetic counseling. If a female is a known carrier of a specific genetic condition, we will provide a copy of the donor’s test reports to the physician and genetic counselor